
Metalyse®
METALYSE® 25 MG IS NOW AVAILABLE FOR
THE FIBRINOLYTIC TREATMENT OF PATIENTS WITH ACUTE
ISCHAEMIC STROKE WITHOUT INTRACRANIAL HEMORRHAGE1
Because Every
Minute Matters

In the AcT phase III clinical trial,
no difference in safety profile was
observed with tenecteplase*
compared to alteplase for the
treatment of AIS.†2
In AcT, the risk of key adverse events including death within 90 days and symptomatic intracerebral haemorrhage was similar for tenecteplase compared to alteplase. Tenecteplase was also similar to alteplase in the rate of additional safety outcomes, including haemorrhagic infarction and intracranial, subarachnoid and intraventricular haemorrhage.2
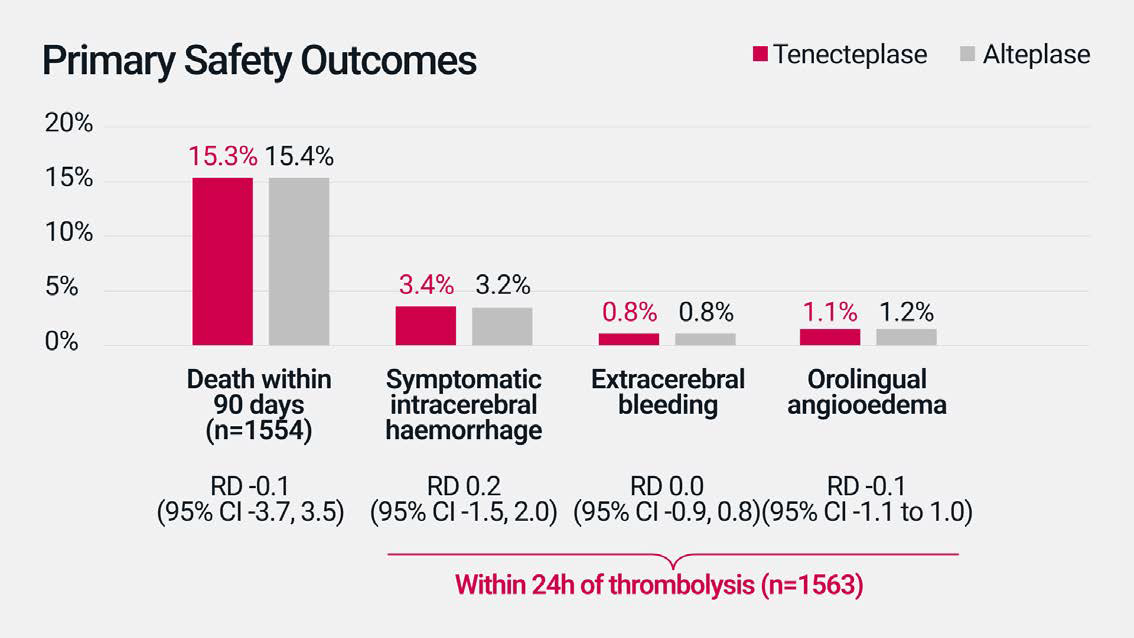
AcT was a multicenter, open-label, parallel-group, registry-linked, RCT, in which 1600 patients were enrolled from 22 primary and comprehensive stroke centres across Canada and randomly assigned to tenecteplase (as a weight-tiered bolus dose, based on 0.25 mg/kg, to a maximum of 25 mg, n=816) or alteplase (0.9 mg/kg to a maximum of 90 mg, n=784). Patients 18 years or older, with a diagnosis of ischaemic stroke causing disabling neurological deficit, presenting within 4.5 hrs of symptom onset, and eligible for thrombolysis per Canadian guidelines, were included. The primary outcome in AcT was a mRS score of 0-1 at 90-120 days after treatment, assessed via blinded review in the ITT population.2
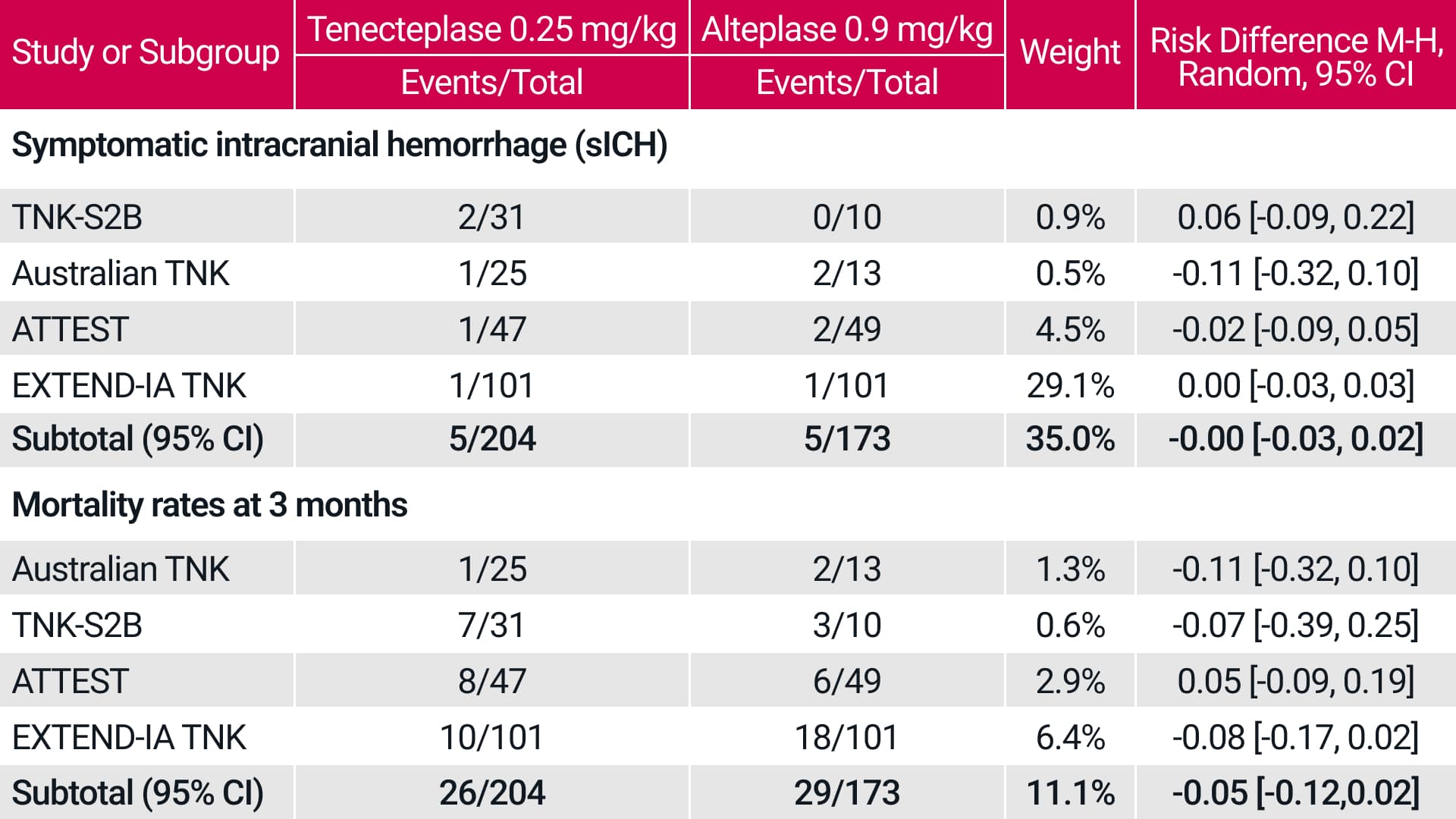
A meta-analysis of 5 RCTs also showed
similar safety outcomes for
tenecteplase vs alteplase in the
treatment of AIS.‡3
In patients receiving tenecteplase 0.25 mg/kg, the meta-
analysis showed similar key safety endpoints for tenecteplase compared to alteplase.
A systematic literature search and formal meta-analysis were conducted per PRISMA guidelines, adapted to noninferiority analysis. The primary outcome was freedom from disability (mRS score, 0–1) at 3-months, and additional efficacy and safety outcomes were analysed. The systematic search identified 5 trials enrolling 1585 patients (tenecteplase =828, alteplase=757). All alteplase patients received standard 0.9 mg/kg dosing, while tenecteplase dosing was 0.1 mg/kg in 6.8%, 0.25 mg/kg in 24.6%, and 0.4 mg/kg in 68.6% of participants.3
Frequency of adverse reactions
System Organ Class | Adverse reaction Frequency |
|---|---|
Immune system disorders (Anaphylactoid reaction) | Rare |
Nervous system disorders (Intracranial haemorrhage) | Uncommon |
Eye disorders (eye haemorrhage) | Uncommon |
Cardiac disorders (Reperfusion arrhythmias) | Uncommon |
Vascular disorders (haemorrhage) | Very common |
Vascular disorders (embolism) | Rare |
Respiratory, thoracicand mediastinal disorders (Epistaxis) | Common |
Respiratory, thoracicand mediastinal disorders (Pulmonary haemorrhage) | Rare |
Gastrointestinal disorders (Gastrointestinal haemorrhage) | Common |
Gastrointestinal disorders (Retroperitoneal haemorrhage) | Uncommon |
Gastrointestinal disorders (Nausea, vomiting) | Not known |
Skin and subcutaneous tissue disorders (Ecchymosis) | Common |
Renal and urinary disorders (Urogenital haemorrhage) | Common |
General disorders and administration site conditions (Injection site haemorrhage) | Common |
Investigations (Blood pressure decreased) | Rare |
Injury, poisoning and procedural complications (Fat embolism) | Not known |
Frequency groupings are defined according to the following convention: very common (≥1/10), common (≥1/100 to <1/10), uncommon
(≥1/1000 to <1/100), rare (≥1/10000 to <1/1000), very rare (<1/10000), not known (cannot be estimated from the available data).
Footnotes
-
*
Tenecteplase was administrated as a one-time decile-weight-tiered bolus dose, based on 0.25 mg/kg for the maximum weight at each tier: <60 kg, 15 mg tenecteplase; ≥60 to <70 kg, 17.5 mg; ≥70 to <80 kg, 20 mg; ≥80 to <90 kg, 22.5 mg; and ≥90 kg, 25 mg.2
-
†
In the AcT phase III clinical trial, the rates of adverse events were similar for tenecteplase compared to alteplase. The main adverse events were: death within 90 days (15.3% for tenecteplase vs. 15.4% for alteplase; RD -0.1 [95% CI -3.7, 3.5]), symptomatic intracerebral haemorrhage (3.4% vs. 3.2%; RD 0.2 [95% CI -1.5, 2.0]), extracranial bleeding (0.8% vs. 0.8%; RD 0.0 [95% CI -0.9, 0.8]), and orolingual angio-oedema (1.1% vs. 1.2%; RD -0.1 [95% CI -1.1, 1.0]).2
-
‡
The safety outcomes analysed were sICH and mortality. Symptomatic hemorrhage events in individual trials were identified using the sICH definition employed in each trial. For mortality and sICH, the noninferiority margins were set at 1%.
-
AIS = acute ischemic stroke, CI= confidence interval, RD= risk difference, sCIH= symptomatic intra cerebral hemorrhage.
References
-
Metalyse® European Summary of Product Characteristics.
-
Menon BK, et al. Lancet 2022; 400:161-169.
-
Burgos A. M. and Saver J. L. Stroke 2019; 50:2156-2162.
